The link between stubble height and Fusarium crown rot infection in subsequent crops is being explored as a practical option for growers to reduce the impact of the disease.
The research is being undertaken by NSW Department of Primary Industries (DPI) research officer Toni Petronaitis, who is completing a PhD through the DPI and GRDC Grains Agronomy and Pathology Partnership (GAPP).
The focus of her three-year research project – with supervision from the NSW DPI’s Dr Steven Simpfendorfer, the University of New England’s Dr David Backhouse and Dr Richard Flavel, and the University of Melbourne’s Dr Graham Brodie – is looking at the survival of the Fusarium crown rot fungus in durum wheat stubble after a chickpea break crop. Clayton Forknall, from Statistics for the Australian Grains Industries (North), is also providing statistical analysis.
“I’m looking to see if we can reduce the amount of Fusarium crown rot inoculum by modifying cereal harvest heights,” Ms Petronaitis says. “We’re trying to determine whether cutting crops shorter can stop the Fusarium crown rot pathogen from continuing to grow in the stubble after harvest.”
Harvest heights
Ms Petronaitis’ research follows her observations of the way in which the fungus builds up on higher stubble.
She started her research during 2019, when seasonal conditions were dry. Accordingly, the harvest heights of stubble she is looking at are 10 to 15, 25 to 35 and 40 to 50 centimetres (see Figure 1).
Figure 1: Harvest-height and harvest-trash treatments implemented in 2019 in durum wheat plots inoculated with Fusarium pseudograminearum. Photos taken at establishment of chickpeas (PBA Seamer) in June 2020 in Narrabri, NSW.

Stubble Kelly-chained after harvest. Photo: Toni Petronaitis

Stubble cut short at harvest. Photo: Toni Petronaitis

Stubble cut to a medium height at harvest. Photo: Toni Petronaitis

Stubble cut tall at harvest. Photo: Toni Petronaitis
“I wanted to simulate short, conventional and stripper front harvest heights,” she says. “Over time, I’ve been looking at how the fungus progresses up the different standing stubble heights.”
For example, in the taller stubble, she says, the fungus grew higher from where it was first detected after the 2019 harvest, probably stimulated by the heavy rainfall in autumn 2020.
She says this suggests the inoculum changes over time. In wet weather, she says, it can move up the stems of retained standing stubble. “We haven’t yet analysed whether we had a higher total pathogen load in the taller stubble, but what I do know is that the pathogen was found higher up in the stubble than where it was detected at harvest.”
When the 2020 chickpea crop was harvested, Ms Petronaitis collected the trash to determine whether different amounts of disease inoculum exited from the rear of the harvester in the retained durum stubble from 2019.
Potentially, she says, where wheat stubble was taller, the harvester may spread some of the Fusarium crown rot inoculum back on to the paddock. Where the wheat stubble was cut short, she does not expect any inoculum was spread during chickpea harvesting.
“Once a harvester spreads the inoculum across the paddock, we can’t use inoculum avoidance strategies like inter-row sowing,” she says.
Another of her treatments is looking at the value of removing trash from the paddock and the effects on Fusarium crown rot inoculum load.
“We’re also looking at whether using a Kelly chain to lightly incorporate the residue has any effect on the Fusarium crown rot load.”
While some may be concerned that shorter stubbles could lead to lower chickpea grain yields, Ms Petronaitis’ research has shown this was not the case for PBA Seamer (PBR) chickpeas at Narrabri, NSW, in 2020.
“The chickpeas grew slightly taller in the shorter stubble and slightly shorter in the longer stubble, but stubble height had no impact on chickpea grain yields. Nonetheless, we can’t rule out that there may be yield differences in more marginal years.”
Pathogen biology
In addition to Fusarium crown rot, Ms Petronaitis is investigating the biology of two other stubble-borne diseases: Common root rot and Yellow leaf spot.
“I wanted to zoom in on these three diseases as they are caused by our most prevalent stubble-borne pathogens in the north, and look at how the pathogens behave in stubble,” she says.
“We know a lot about these pathogens when they’re causing disease, but we don’t know much about what they do in the stubble after a wheat plant dies.”
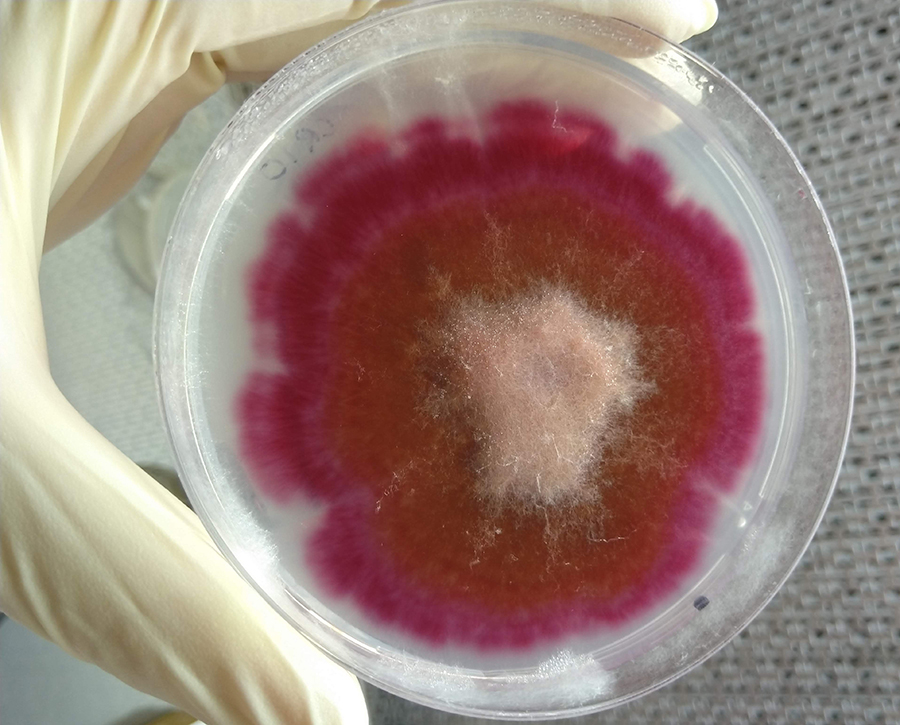
A fungal isolate of Fusarium pseudograminearum, one of the pathogens that causes Fusarium crown rot. Photo: Toni Petronaitis
Although many people assume the inoculum is dormant in stubble while waiting for the next cereal crop, if there is moisture in the environment the pathogens remain active, she says.
One of her experiments looked at how much moisture each of the pathogens need to start growing in stubble, to see whether one is more competitive than the others. She also determined how fast each pathogen grew within different types of cereal stubble.
“We grow varieties with different levels of resistance to various pathogens, so I wanted to look at whether that makes a difference to pathogen activity once the plant dies and stubble remains. Disease-causing fungi could colonise different types of stubble in the same way. It didn’t matter what variety or crop type it was to begin with, which included oats, barley, bread wheat and durum wheat.”
Another interesting finding, she says, was that the Fusarium crown rot pathogen can start growing when less moisture is available compared to Common root rot and Yellow leaf spot.
“The Fusarium crown rot fungus seems to have a competitive advantage in drier environments and can start growing in stubble at 92.5 per cent relative humidity, under lower moisture conditions than the other two pathogens, which didn’t show any growth until 97.5 per cent relative humidity.
“Fusarium crown rot is also able to grow more quickly than the fungi that cause Common root rot and Yellow leaf spot, so its capacity to grow in stubble appeared to be a lot better than the other two pathogens.”
Further glasshouse experiments will look at how different levels of Fusarium crown rot infection during the growing phase of wheat affects the pathogen’s post-harvest growth.
A persistent problem
While Fusarium crown rot generally seems a bigger issue in drier years, Ms Petronaitis says the pathogen can also be present in infected stubble in wetter seasons.
“It’s frequently there. We only see the effects in drier years because the plant becomes stressed in low-moisture conditions. That’s when Fusarium crown rot becomes a problem for the plant’s vascular system,” she says.
“When water levels are low and/or hot conditions occur during grain filling, that’s when we tend to see expression of the disease as white heads and shrivelled grain. However, even in wet years, if you look at the stems of infected plants, a lot of the time you can find some basal browning at the bottom of the plant indicative of Fusarium crown rot infection.”
She encourages checking for the characteristic basal browning and sending stubble to NSW DPI if growers are not sure. “There’s also PREDICTA® B diagnostics, which will pick up not just Fusarium crown rot levels, but also a wide range of other cereal pathogens,” she says.
“When you’ve had a good year with lots of rain it can be really difficult to tell whether there is inoculum in the paddock. It’s always best to be safe and to check before you sow.”
She cautions growers against sowing wheat after wheat because the risk for Fusarium crown rot may be increased. “If you are thinking of growing wheat or barley in 2021 after growing cereals in 2020, test to determine your risk of disease,” she says. “It may be wiser to grow a break crop if disease inoculum levels are high.”
Microwave radiation
Ms Petronaitis is also exploring the value of microwave radiation for killing the Fusarium crown rot pathogen in stubble. Preliminary work, using a domestic microwave oven, showed that microwaving infected durum stubble caused the pathogen to die.
“As part of my PhD, I’ve been microwaving the spores of two different species of Fusarium, which cause Fusarium crown rot, and the Common root rot pathogen,” she says.
“The good news is they can be killed with microwave energy and they need relatively small amounts to kill them.”
It is likely one energy dose will kill multiple pathogens even if they have very different spore structures, she says. While the results are promising, her next step is to determine whether microwave energy is an efficient way to kill pathogens in stubble.
More information: Toni Petronaitis, 02 6763 1219, toni.petronaitis@dpi.nsw.gov.au

























































