Key points
- Soil water repellency is linked to organic matter composition
- Very small amounts of specific compounds cause water repellency
- Computer modelling of the soil, organic compounds and water system can help identify why compounds behave in a particular way
- Ways to modify soil organic matter to switch off or modify the culprit compounds are being investigated in a targeted way
Professor David Henry’s team is on the hunt for the culprits that cause water repellency in Western Australian soils to help inform the development of novel ameliorants.
Soil water repellency is widespread across southern Australian soils, affecting at least five million hectares of agricultural soil. It is estimated to cause at least $300 million in lost productivity each year. This is happening due to uneven wetting of soils, run-off and leaching of fertiliser, flooding and erosion. It results in gaps in crop rows, erratic crop emergence and dry patches across and down the soil profile.
More common in the top 10 centimetres and on sandy soils, sandy gravels and forest gravels, non-wetting causes problems from the start of the season and can disrupt management for the rest of the year. Operations such as spraying and fertilising paddocks produce suboptimal results when plants are at different growth stages because of non-wetting issues.
Long-term solutions to non-wetting include liming soils or physically inverting, mixing and claying soils by ploughing, spading, delving and clay spreading. These are expensive options and it is unlikely growers can implement them across the whole non-wetting area in one or even several seasons, especially if they have large areas of non-wetting soils.
Wetting agents are shorter-term and cheaper solutions that can be used on some parts of the farm while growers simultaneously fix non-wetting issues using longer-term approaches on other areas of a property. The shorter-term options are useful for getting the crop out of the ground in the season they are implemented. The downside is that results can be inconsistent and treatment might need to be repeated every year or two.
Is there a better solution to managing water repellent soils that could be cheaper and more sustainable?
This is the question that Professor David Henry from Murdoch University is turning his skill set to. As an expert in inorganic and computational chemistry, his work has encompassed a wide range of applications including pollution remediation, nanomaterials for delivering therapeutic drugs in oncology and organic–inorganic interactions in soil.
Professor Henry’s work began with support from GRDC through a large Department of Primary Industries and Regional Development led project ‘Delivering enhanced strategies for improved crop performance on water’ between 2014 and 2019.
This project had three broad aims:
- to undertake fundamental and applied research to increase knowledge of soil repellence in WA cropping systems
- deliver an extension strategy to growers and agronomists to support improved capacity to select the best management strategies for repellent soils, and
- build capacity for WA through training of PhD students, a post-doctoral fellow and regionally based graduates
Developing a keen interest in a high priority WA soil constraint, Professor Henry has continued the work with colleagues with support from the Cooperative Research Centre for High Performance Soils (Soils CRC) and Murdoch University.
“My team has been looking at extracting, isolating and identifying the compounds and molecules that create water repellent soils to better understand hydrophobicity,” Professor Henry says.
We are using traditional organic chemistry analysis to identify the suspect molecules and then we ‘rebuild’ the environment virtually using computational chemistry or modelling to create simulation scenarios.
“This breaks the soil-water system down to single factors and we can start learning about how the culprit suspects behave and interact with the inorganic soil matrix and then build the system back up one factor at a time.”
The water repellent culprits
Specific groups of organic molecules from plant matter are the main culprits. These compounds enter the soil as plant matter decomposes but the amounts released are plant species-dependent.
“We have shown that leaves, litter and even roots of eucalypts and other native species contribute non-wetting compounds to the soil in native environments. Likewise, crop residue is a significant contributor in agricultural settings. We are currently assessing how much variation there is between the residue of different cereal crops for inducing water repellency,” Professor Henry says.
Professor Henry’s team has also investigated how heating soil increases water repellency and how this is linked to the endemic plant species in the area.
“This tallies with the field situation, as repellency develops over summer and breaks down over winter through microbial action. However, the higher levels of repellency during summer have implications for dry seeding and the efficacy of herbicides,” he says.
“Professor Richard Harper and I, working with support from the Soils CRC,have looked at faster ways of assessing soil in the field using near-infrared spectroscopy, electromagnetic and gamma-radiometrics to provide farm-scale information to growers but field calibration has proved challenging.
“However, we now have faster laboratory techniques for assessing field samples. We are using accelerated solvent extraction to extract organic matter, running the extracts through a gas chromatograph to isolate compounds and then a mass spectrometer to identify these compounds.”
The team has found that there are about 120 compounds of interest, but this was honed down to 19 that appear to be closely linked to soil water repellency. The main culprits were identified as amphiphilic, long-chain organic compounds including acids, amides and alcohols, as well as a range of sterols. The key features of these compounds are the polar end group and long hydrophobic tails that create the water repellency.
Water repellency persists on sandy soils until clay content is greater than five to 10 per cent.
Figure 1: Computer model showing distribution of amphiphilic molecules that cause water repellency on (a) sand and (b) clay.
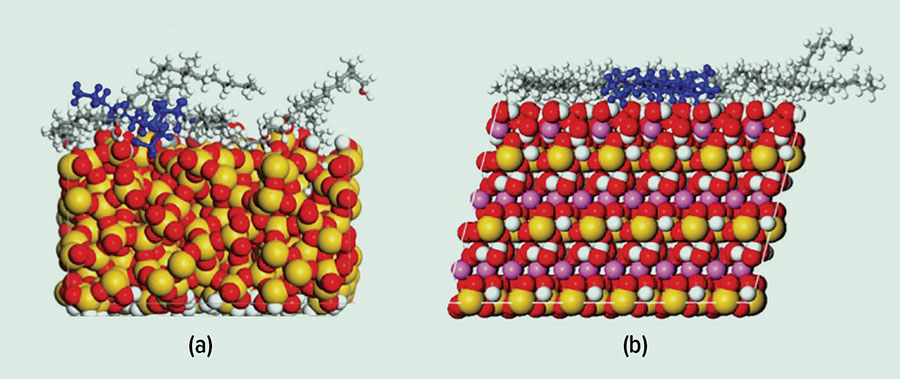
Source: Professor David Henry
This prompted a further question – is this just about particle size or is there important chemistry also involved?
“To investigate what is going on at a molecular level, we build computer models of sand and clay together with models of the amphiphilic, organic molecules. We then run super-computer simulations of these models under environmental conditions. From these simulations, we learn how and why certain organic molecules induce water repellency and how other compounds can enhance or decrease the observed non-wetting,” Professor Henry says.
Through these computer simulations, we have learnt that the way molecules organise and arrange themselves varies on different minerals, as does the way they interact with each other. Therefore, the mineral chemistry is just as important as the mineral particle size.
The combination of experiment and simulation has revealed that some organic compounds contribute to water repellency, some lower non-wetting and others have no effect at all.
“We have found that palmitic acid, a 16-carbon chain organic molecule, is one of the most effective at inducing water repellency even at tiny concentrations in the soil.
“Our findings are pointing to the fact that if we can reduce the number of these culprit compounds, we can alleviate water repellency. Our current research is looking at novel amendments to bind the culprit molecules or assist natural processes to convert them to benign species.”
More information: Professor David Henry, D.Henry@murdoch.edu.au, 08 9360 2681

























































