Outbreaks of Turnip yellow virus (TuYV) in canola and pulse crops can cost the Australian grains industry millions of dollars in lost yield.
TuYV can be particularly destructive in pulses, since some crops – such as field peas and lentils – are often asymptomatic, yet when tested can be 100 per cent infected and see yield losses at harvest time of up to 50 per cent.
Until recently, the virus has been difficult to monitor and research, particularly in pulses, since it is often found co-infecting plants with similar viruses and various mosaic viruses.
In canola, the yield losses are similar to pulse crops, with more severe losses occurring during the rosette growth phase until stem elongation. The virus can also affect the oil and fatty acid content of the seed.
Carried by green peach aphids into a crop as early as only a few weeks after germination, the virus can infect a plant at any time and, once infected, it remains systemic in the plant for its life span.
Weeds and volunteer crop plants from green bridges allow aphids and virus to survive between cropping periods. Outbreaks of the virus have been linked to wet summers and autumns that result in larger weed and plant loadings going into the seeding period.
In a GRDC-invested global research collaboration, researchers across Australia and at the International Center for Agricultural Research in the Dry Areas (ICARDA) are combing through thousands of germplasm samples in the hope of finding resistant genetics that will allow crops to avoid yield losses from TuYV and other related viruses.
Australian program manager and New South Wales Department of Primary Industries plant pathologist Joop van Leur says the global search for industry solutions will continue until solutions for growers are found.
“There are some positive indications that some resistance germplasm may have been uncovered for lentils, but it is still early days,” Mr van Leur says.
“Chickpeas are proving particularly problematic because, until recently, we have not had the diagnostic tools to be able to differentiate between the various virus strains that one plant may be hosting, meaning it is then difficult to know which resistant gene we need to be searching for.”
The ICARDA research facility in Tunisia manages the world’s largest collection of chickpea and lentil germplasm, allowing for concentrated research into the genetics of these specific crops.
The research project in Australia includes work from Western Australia, Victoria, New South Wales and Queensland.
Canola
Canola plants infected with TuYV can develop distorted and discoloured leaves and the plant may also have stunted growth, but asymptomatic infection can also occur.
The most devastating documented outbreak of TuYV occurred in South Australia in 2014, where researchers estimated up to 10,000 hectares were destroyed by the virus, resulting in large yield losses at harvest time.
It was estimated only 20 to 25 per cent of crops in the lower and mid-north of SA escaped infection.
In Esperance, on WA’s south-east coast, widespread early TuYV infection contributed to the disappointing average yield of 1.43 t/ha across the 210,000ha of canola crops in 2018.
In responding to the urgent need to find solutions in canola, researchers in WA have screened a wide range of canola and other brassica cultivars, including most commercially available Australian canola varieties, in the search for a host gene resistant to TuYV.
While Australian commercially available canola varieties are currently susceptible to the virus, the good news for canola growers is the discovery of resistance in three older rapeseed lines (one from Germany and two from China), which could provide breeders with materials to create resistant varieties.

Dr Ben Congdon looks for Turnip yellow virus in the canola plants. Photo: Evan Collis
Department of Primary Industries and Regional Development (DPIRD) research scientist Ben Congdon, who managed the WA research arm of the GRDC-invested project, inoculated hundreds of canola varieties with TuYV both in the field and in glasshouse conditions, and subsequently screened them for resistance.
“Except for ATR Stingray and older varieties Trigold and ATR-Stubby, which exhibited moderate resistance to TuYV infection, all Australian genotypes we tested were highly susceptible,” Dr Congdon says.
He says the discovery of the resistance gene in these three older lines of rapeseed (which are quite different genetically to modern-day canola varieties) presents an exciting opportunity for the canola industry.
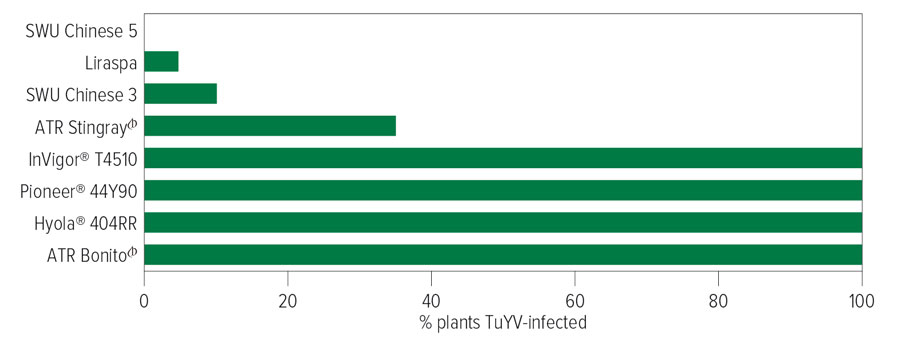
Figure 1: Percentage of canola plants infected with TuYV following inoculation with green peach aphids. Source: DPIRD.
“This is an incredibly timely discovery, particularly given we are running out of effective insecticide chemistries to control the green peach aphid.
“Green peach aphid is the most resistant insect in the world and while neonicotinoid seed treatments have previously offered good protection, resistance to these treatments is a developing threat and the likelihood of deregistration is increasing with Europe recently banning these chemicals in all field crops.”
Dr Congdon says his research will add to the global knowledge about the virus, since TuYV is a serious threat in Europe.
“In response to the damage caused by TuYV in Europe, a resistant gene from a resynthesised line has been incorporated into the genetics of several canola varieties, but they are totally reliant on this one gene,” he says.
“Since these newly discovered resistant genetics come from two semi-winter lines and one spring line, our screening program will add diversity to this global gene pool.”
 Researchers have discovered a resistant gene in three older lines of rapeseed. Photo: Evan Collis
Researchers have discovered a resistant gene in three older lines of rapeseed. Photo: Evan Collis
Further research investigating the impact of heat stress on the resistance genes has shown while there was an increase in infection rates at very hot temperatures, resistance to virus accumulation in the tissue was maintained.
“Temperature appears to be an important variable for expression of infection resistance, but the true test will be how these resistance genetics perform under field conditions as part of an integrated disease management strategy,” Dr Congdon says.
Pulses
A wet autumn period in some parts of Victoria may have set the scene for near-perfect TuYV conditions for pulse crops in the state.
Agriculture Victoria plant disease specialist Piotr Trebicki says the virus is of huge concern to the industry, particularly since symptoms are not obvious on some legumes, such as lentils.
Conversely, chickpea plants show obvious symptoms, such as leaves turning yellow (kabuli type) and red (desi type), with the plants often killed by the virus.
Dr Trebicki says TuYV can be as devastating to legume crops as it is to canola. “We have the very difficult situation where crops, such as faba beans, field peas and lentils, do not display symptoms yet can be 100 per cent infected with the disease,” he says.
He says trials in Horsham on field peas and lentils have supported this concern, with inoculated but asymptomatic plants seeing yield losses of 40 to 50 per cent at harvest.
“As researchers, even we were not convinced that our inoculation method had been effective, since there were no symptoms at all in the plant,” Dr Trebicki says.
“So we tested those plants to make sure we had completed the inoculation correctly, and the plants were 100 per cent infected with TuYV.”
He says Agriculture Victoria is attempting to educate growers on the importance of managing the green bridge over autumn – and to have clean paddocks prior to seeding – as one way to manage the disease.
“Unfortunately, when you don’t see symptoms in a plant, it can be easy to assume your crops have escaped the virus, but we know that this year can be particularly challenging because of the amount of TuYV-infected volunteer plants we have seen around the region prior to the seeding period, which can spill into the crops.”
Further research
Researchers in Queensland have also been part of this wide-ranging study, assisting in the development of diagnostic tools and screening methods.
Queensland Department of Agriculture and Fisheries (DAF) principal plant pathologist Murray Sharman says until recently, it was believed the virus attacking pulse crops was Beet western yellow virus, (BWYV), but it is now understood to be complex TuYV strains or newly recognised viruses in the same virus family.
Newly developed diagnostics by Dr Fiona Filardo (DAF) and Dr Sharman have greatly improved the industry’s understanding of the viruses affecting pulses in the course of this research project.
Dr Sharman says this discovery has highlighted the diversity of viruses that can infect pulse crops around the world.
“While this does complicate our research going forward in terms of isolating the genes required to resist these different viruses and strains, it is important for growers because it means we now have a more accurate picture of what is happening in the paddocks and can channel our research in more specific directions,” Dr Sharman says.
He says field observations also suggest that pulse crop proximity to infected canola may affect infection rates. “We have suspected there could be interactions between an aphid infestation in a TuYV-infected canola crop and subsequent TuYV outbreaks in nearby pulse crops. If conditions are right and the time of canola crop maturity is favourable for aphids, they may migrate en masse, moving into a pulse crop in a nearby location.
“Aphids rarely colonise chickpeas but can feed long enough to cause virus infection. The pattern of infection in chickpeas indicates that there is a one-way flow of infectious aphids moving from nearby hosts such as weeds or other crops into chickpeas.”
More information: Joop van Leur, joop.vanleur@dpi.nsw.gov.au; Ben Congdon, benjamin.congdon@dpird.wa.gov.au

























































