Resistance monitoring of key insect pests has shown that combined grain protectants are a valuable integrated pest management tool for stored grain.
Up to 30 per cent of freshly harvested grain is treated with contact insecticides – or grain protectants – before being placed into storage.
In the 1990s the development of multiple resistances to protectants in key pests, such as the lesser grain borer and red flour beetle, saw the introduction of binary applications.
Today, a triple combination of spinosad, chlorpyrifos-methyl and s-methoprene is used to control the entire spectrum of pest species that have developed resistance to one or two of these treatments. However, individual grain protectants are still used where multiple resistances are not present.
To help growers select effective protectants, the status of resistance to grain protectants in major pest species has been updated as part of an ongoing national GRDC investment in resistance monitoring for insect pests of stored grain.
The study, undertaken by researchers from the Queensland Department of Agriculture and Fisheries and NSW Department of Primary Industries, has reaffirmed the value of using combined treatments to overcome multiple resistances in key pest species. The results also highlighted that some of the individual treatments are still effective in managing some species.
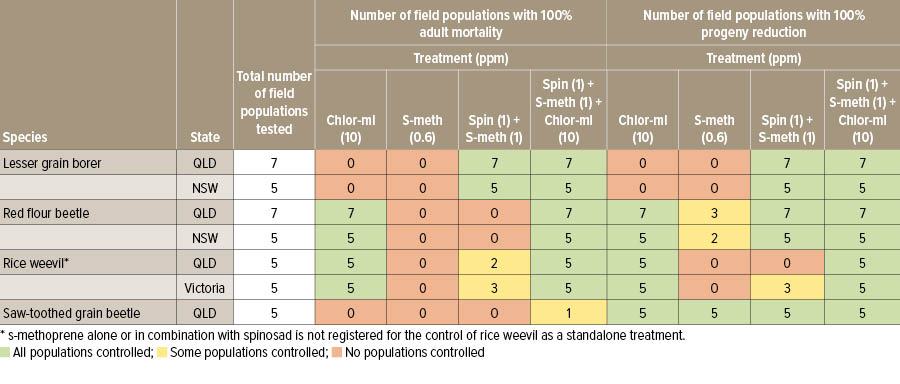
Field populations of four major pest species were collected from farm storages in Queensland, NSW and Victoria and tested for resistance. The 12 populations each of lesser grain borer and red flour beetle, 10 populations of rice weevil and five populations of saw-toothed grain beetle were tested in wheat treated with the protectants listed in Table 1.
Note that s-methoprene alone or in combination with spinosad is not registered for the control of rice weevil as a standalone treatment.
Table 1: Number of field populations with 100 per cent adult mortality or progeny reduction in wheat treated with currently registered grain protectants: chlorpyrifos-methyl (chlor-ml), s-methoprene (s-meth), and spinosad (spin).
Source: Dr Manoj Nayak
Results
The reduction in number of adult progeny was the main criterion for success, but adult mortality was also measured.
The triple combination was 100 per cent effective in progeny suppression against all four species tested. Lesser grain borer has developed resistance to several grain protectants in the past and needs to be watched carefully; however, results to date show that spinosad is highly effective against this species and resistance is less likely to develop.
The binary treatment of s-methoprene and spinosad delivered complete suppression of progenies in all populations, except for rice weevil. This combination is not registered for standalone control of rice weevil. In adults it only controlled lesser grain borer, most likely due to the spinosad.
Chlorpyrifos-methyl alone was ineffective against lesser grain borer, reflecting its long-standing resistance to organophosphate protectants. It successfully prevented progeny production in all other species but had poor control of saw-toothed grain beetle adults.
The growth regulator, s-methoprene, was ineffective against adults of all species tested, as expected. Resistance has been known to occur in lesser grain borer for many years and s-methoprene is not registered for standalone control of rice weevil. It achieved complete suppression of progenies in all populations of saw-toothed grain beetle and five of the 12 populations of red flour beetle.
Growers should continue to monitor for live insects in treated grain and, if detected, send to the nearest research laboratory for resistance testing or contact an expert via 1800 WEEVIL.
More information: Dr Manoj Nayak, 0421 225 906, manoj.nayak@daf.qld.gov.au

























































