Chickpeas are notoriously fussy about their choice in rhizobia partner, but a new research effort is looking to find a better match to enhance and expand chickpea production.
Investment is increasing in developing high-value pulse crops for Western Australian growers, in particular chickpeas. But the potential area of production for chickpeas in WA is restricted by the prevalence of acid soils.
Together with increased efforts to breed acid-tolerant chickpea varieties, it is critical to identify improved rhizobia strains that can expand chickpea production into these challenging soils and improve performance of the pulse more broadly in the state.
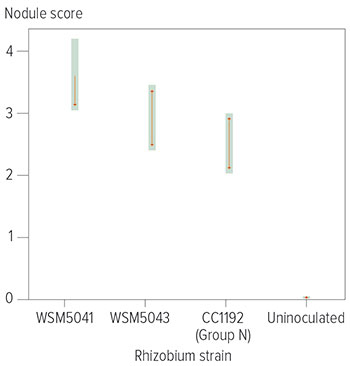
Figure 1: Average nodule score on chickpeas at 10 weeks of growth. Centre points represent estimated marginal mean, shading represents 95 per cent confidence intervals and arrows indicate Tukey comparison values.
Source: Murdoch University
Chickpea nitrogen fixation efficiency is reported to be lower than other pulses, and recent trials in acidic WA soils have revealed reduced nodulation (Figure 1).
This is unsurprising as the inoculant strain has not been updated for 40 years.
With GRDC investment, three regional Nitrogen Fixation Program (NFP) projects are focusing on extending the adaptation and reliability of pulse crops, including chickpeas, through releasing more-robust inoculant strains and improving inoculation practices.
The NFP brings together research teams from Legume Rhizobium Sciences at Murdoch University, the WA Department of Primary Industries and Regional Development, the South Australian Research and Development Institute, the University of Adelaide and the New South Wales Department of Primary Industries.
The Murdoch University NFP team, led by Dr Ron Yates, is taking a two-pronged approach to learn about the genetics of rhizobia specific for chickpeas and using this knowledge to see whether they can find a better strain to enhance and expand chickpea production.
Environmental audit
Chickpeas originate from more alkaline soils in the Middle East and, in the past for other pulses, expeditions have returned to the crops’ centre of origin to see whether better-performing rhizobia can be found for crops grown in Australian soils. However, there might be potential to find a better-adapted rhizobia for chickpeas in Australia.
Dr Yvette Hill from Murdoch University has been undertaking environmental surveys to assess the status of variability of chickpea rhizobia in WA, more recently extending to Queensland and NSW.
“Nitrogen fixed by rhizobia is fundamental to sustainable farming practices and healthy productive pulse crops provide many benefits in cropping sequences, not only in the provision of nitrogen but as a disease and weed break,” Dr Hill says.
There are thousands of strains of rhizobia that can nodulate and potentially fix nitrogen with each pulse host. However, the amount of nitrogen fixed can vary substantially, depending on the compatibility of legume host and rhizobial strain.
Inoculant rhizobia strains are members of a number of bacterial genera – Bradyrhizobium, Mesorhizobium, Methylobacterium, Rhizobium and Sinorhizobium. They are categorised into inoculant groups based on the legumes they nodulate. Some groups are quite promiscuous and can form symbiotic relationships with several different pulses.
Chickpeas, however, form an association specifically with Group N rhizobia – Mesorhizobium ciceri, also known as strain CC1192, a strain originally from Israel and introduced as the inoculant in the 1970s.
Bacteria evolve over time and their genetic makeup can change to make them better-adapted to their environments. This means there is potential to find more-efficient nitrogen fixing strains to pair with pulses. However, for chickpeas there has been no dedicated efforts to upgrade the rhizobia partner since the 1970s.
“We know that Mesorhizobium are particularly adept at genetic change but we needed to learn just to what extent,” Dr Hill says.
To this end, she has been conducting surveys of Mesorhizobium ciceri across WA, Queensland and NSW cropping environments using genome sequencing techniques to track genetic differences.
“We are finding a range of differences as these bacteria are able to exchange the sections of their DNA that encode the ability to symbiotically fix nitrogen.
We have been able to identify strains evolved in Australian soils that appear superior to the present Group N strain used to inoculate chickpeas and they are now being evaluated in field trials in WA, SA and NSW.
Field performance
Potential new rhizobia strains need to be tested for their compatibility with any new chickpea varieties and in target production environments.
A number of these newly evolved Mesorhizobium strains that had been collected from WA acidic soils during the surveys are being trialled at Goomalling with the chickpea variety CBA Captain , after showing promising results from an acidic soil establishment trial conducted at Northam during 2020-21. The nodulation of WSM5041 is significantly greater than Group N (CC1192), with double the number of nodules at 10 weeks in soil of pH 4.9.
Further testing of new chickpea rhizobia strains will be required to determine their compatibility with farming system practices and new cultivars being developed. For example, applied herbicides and herbicide residues might reduce chickpea nodulation, nitrogen fixation and yield. Similar effects might occur with seed dressings such as applied fungicides and trace elements.
Evolution in the field
Ideally, rhizobia need to be able to survive and grow in soil in the absence of the host legume plant for several years, but for chickpeas this is not the case and seed has to be inoculated at each sowing.
Dr Hill has set up a number of field trials at South Burracoppin, WA in acidic soils where chickpeas had not previously been grown. Treatments include inoculated chickpeas, uninoculated chickpeas and wheat. The influence of these treatments is being monitored for changes in the evolution of chickpea-nodulating strains over time. Dr Hill is planning to extend this research for the next decade.

Aerial view of the Goomalling chickpea rhizobia site testing new rhizobia on CBA Captain (PBR) chickpeas. Photo: Benedict Arthur, Murdoch University
“Essentially, it is a more-controlled version of the evolution of the strain we are seeing across Australia but will give us greater understanding of how inoculant strains influence the population of nodulating bacteria in our soils,” Dr Hill says.
At these sites, there is the opportunity to study the pre-existing, non-symbiotic bacteria present in our soils that are capable of acquiring symbiotic DNA from inoculant strains becoming new rhizobia to nodulate chickpeas. These new strains are better-adapted to the conditions and this might improve survivability as an inoculant or in the soil.
Adding to the rhizobia library
Any new rhizobia strains will be captured and stored in the purpose-built International Legume Inoculant Genebank supported by GRDC at Murdoch University.

Dr Jason Terpolilli with a treasure trove of rhizobia carefully stored in the purpose-built GRDC-supported International Legume Inoculant Genebank. Photo: Dr Sue Knights
Dr Jason Terpolilli says this is a unique international resource where rhizobia strains are carefully catalogued and available for further scientific and commercial use.
The samples are effectively fixed genetically at a point in time so we preserve them. It provides a unique genetic resource that we can go back and mine.
“The collection houses more than 11,000 rhizobia strains collected from 59 countries from 531 legume species. This includes the current commercial inoculant strains and their back-ups. We are completing the full genome sequences of all the commercial inoculant strains. These sequences will enable us to monitor how inoculant strains for other legumes are performing in the field, just like we are doing for chickpeas.”
More information: Dr Yvette Hill, 08 9360 6731, y.hill@murdoch.edu.au

























































