Key points
- Chickpea Breeding Australia has been operating for more than a year
- The five-year, $30-million breeding program has hit the ground running and is engaging at pre-breeding, breeding and farming system levels to increase chickpea production
- Key areas include improving yields, cold tolerance, disease resistance and adaptation to soils with a low pH
- Wild relatives, collected in a previous GRDC project almost a decade ago in 2013, are playing an important role
By doubling plot numbers, disrupting disease pathways and incorporating the best traits from wild sources, Chickpea Breeding Australia (CBA) has hit the ground running.
The five-year, $30-million breeding program, launched in early 2020, is working to expand the geographic reach of chickpeas from their traditional northern region stronghold into central and southern New South Wales and Western Australia.
CBA is a collaboration between GRDC and New South Wales Government and aims to deliver for growers across the nation through a focus on new traits for expansion areas and improved varieties for traditional growing areas.
CBA program leader and NSW Department of Primary Industries (DPI) chickpea breeder Dr Kristy Hobson says the team is engaging at every possible angle to improve yields, cold tolerance, disease resistance and adaptation to certain soils – constraints that have held chickpea production back.
Trials
The program’s first variety – CBA Captain, released in 2020 – is already showing what is possible. Dr Nathan Craig, the executive officer of WA’s West Midlands Group, has run trials and field days with CBA Captain and is impressed.
“CBA Captain is awesome when compared to PBA Striker. It has more vigour, is healthier and seems more adapted already. I am pretty confident that there is a great future in WA with chickpeas and CBA Captain is part of that.”
Time-of-sowing trials have also shown how well chickpeas perform when planted a month earlier, in mid-April. Last year, the West Midlands Group trials with varieties including PBA Striker saw yields double from one tonne per hectare to 2t/ha. The numbers on CBA Captain have not been crunched yet, but Dr Craig expects that variety to perform just as well.
CBA has more than doubled trial plot numbers from 1416 to 3540 in the past two years, with yield trials underway across the WA wheatbelt.

Trials of CBA Captain have been outstanding, according to Dr Nathan Craig, executive officer of the West Midlands Group. This image shows the new chickpea variety growing at a collaborator’s trial, the Facey Group’s site near Wickepin. It was sown in mid-April and this image was taken in mid-August. Supplied: Amy Bowden, Facey Group
Trials at Mingenew, Dalwallinu, and Cunderdin are a mix of stages one to three desi varieties. Run by Living Farm, more than 3300 plots were planted during 2020 and 2021. The Department of Primary Industries and Regional Development (DPIRD) has been responsible for 192 plots of stage 3 desi types at Scaddan, north of Esperance.
Living Farm managing director Richard Devlin says the trials are interesting not only because of the new germplasm being tested but also because of the geographical push.
“Traditionally, chickpeas have been grown in the far eastern or northern wheatbelt. Kristy (Hobson) is pushing them more into the central and southern wheatbelt region with great results. From what we see coming across the header deck, these varieties are doing well in terms of yield and harvestability.”
Mr Devlin says this geographic push gives growers new legume options. “At the moment, chickpeas are double the price of lupins. That’s a fair incentive to get it right. So, if we get the yield and agronomics right, such as harvestability and disease tolerance, there will be a high uptake in the southern region.”
Based at Esperance, WA pulse specialist and DPIRD research scientist Mark Seymour says the challenges to growing chickpeas in non-traditional areas include risks from diseases such as Ascochyta blight, cold tolerance and adaptation to soils that can contain boron and salt or high acidity.
“There is interest in chickpeas but there is also competition from other crops. That’s why we are all working to address these issues.”
The approach includes work in pre-breeding, breeding and in farming systems with teams from Curtin University, CSIRO, DPIRD and Murdoch University. Some of the projects being worked on are included below.
Disease disruption
To increase Ascochyta blight resistance, Curtin University’s Dr Robert Lee is investigating the interaction between the pathogen and the chickpea plant.
The research, at the Centre for Crop and Disease Management (CCDM), a co-investment by GRDC and Curtin University, involves an in-depth molecular exploration of the pathogen’s arsenal.

Curtin University’s Dr Robert Lee is investigating the interaction between the pathogen and the chickpea plant. Photo: Evan Collis
Studying the Ascochyta rabiei pathogen, which causes Ascochyta blight in chickpeas, he and his team has found that its genome has important differences when compared to other plant pathogens – that is, effector genes.
“Effector proteins are unique; they do not have similar DNA or protein sequence elsewhere in nature. The interaction between the pathogen effectors and the host plant receptors can lead to a hypersensitive response. This can mean plant cell death and necrosis – for example, the brown lesions that we see on diseased plants.”
A long-term goal is to discover which chickpea receptor genes interact with the pathogen’s effector genes. “Any variation in chickpea germplasm, including wild chickpea species, will provide opportunities to develop varieties where the pathogen effectors are not recognised.”
Disrupting the interaction between pathogen and host provides a way to increase disease resistance. “Experience shows that varieties with good resistance can soon be affected by aggressive isolates. Our focus is to use genomics to understand the genes that are expressed during infection. That way we can get a handle on what types of genes might be contributing to increased aggressiveness.”
Annual isolate collections take place to keep up with an ever-evolving enemy. “Although chickpea production in WA is fairly limited to some discrete areas, we are collecting WA isolates, characterising them, putting them in the national collection, and assessing them for their aggressiveness levels.”
Up until 2019, Dr Lee says, WA had no evidence of the highly aggressive isolates seen in the eastern states. This work with WA isolates has also helped inform growers about CBA Captain’s disease rating. “It shows that some of the research we have done has already fed into the advice given around a new variety.”
Wild relatives
While Dr Lee concentrates on the pathogen, his colleague Dr Lars Kamphuis is working to better understand the resistance found in wild chickpeas.
“By making crosses with wild chickpeas, our aim is to bring more genetic diversity as well as disease resistance into our domestic lines.”
Collected by CSIRO’s Dr Jens Berger in south-eastern Turkey in 2013, the first set of wild relatives was evaluated for Ascochyta blight resistance in 2015, the second set in 2018.
Using moderately aggressive and highly aggressive Ascochyta blight isolates, and an isolate mix, material was found that performed better than or similar to varieties in Australia, Dr Kamphuis says.
“There are about 30 that seem to be on par with responses that we see in current varieties, but the hypothesis is that they would be different sources of resistance to those currently in the breeding program.

Researcher Dr Lars Kamphuis is working to better understand the resistance found in wild chickpeas. Photo: Evan Collis
“Our next objective is to develop segregating populations to understand what parts of the wild chickpea genome provide resistance, so this resistance can be introduced into the breeding program.”
Linked to this is another project with the International Center for Agricultural Research in the Dry Areas (ICARDA) to develop an international chickpea set that can distinguish different isolates’ aggressiveness. Curtin University’s role is to identify which genes in the pathogen are responsible for what is observed in the plant.
The emerging disease Sclerotinia sclerotiorum is also being researched. Sclerotinia sclerotiorum is often found when chickpeas are grown in rotation with canola, the case in expansion areas such as WA.
Former PhD student Dr Virginia Mwape in the CCDM team developed a disease assay to screen domestic and wild species and identify Sclerotinia resistance.
The work has found that while PBA HatTrick had the most robust levels of resistance, that resistance comes from many regions of the genome.
“From a breeding perspective, that makes breeding for resistance more difficult,” Dr Kamphuis says. “However, there are modern breeding techniques we can use to bring those complex traits in, including genomic selection. There are also wild sources we found with some resistance to Sclerotinia that we can bring in. We just don’t understand the underlying resistance yet – that is a future opportunity.”
Cold tolerance
The wild germplasm collected by Dr Berger is also being used in cold tolerance work.
Dr Berger says that, over time, chickpeas have lost their ability to deal with colder weather. “This an issue not just in southern Australia but in all Mediterranean climates.”
He says addressing this would improve pod set and yield. “Australian growers want to grow chickpeas as a winter annual. Sowing earlier has the potential to reduce the risk of terminal drought later in the season but can make it too cold for pod set.
“We have found some material that has cold tolerance and starts podding earlier – the problem we want to solve. Now we want to find out more about what causes that cold tolerance and breed that into commercial varieties.”

Over time, chickpeas have lost their ability to deal with colder weather. Addressing this would improve pod set and yield. Photo: Evan Collis
This includes a novel project to understand the physiology behind cold tolerance. “It is funny – everyone accepts that 15°C or less is a problem, but we really don’t know why.”
The new work will aim to uncover this, while designing a screening process.
Postdoctoral student Olive Onyemaobi is working on the hypothesis that chickpeas’ lipid profile changes as the temperature decreases.
In simple terms, what happens with plant cell membranes under cold stress can be thought of as similar to how butter and margarine react when cold.
Butter is made up of higher levels of saturated fat. When it is refrigerated and gets colder, its surface hardens, making it is more difficult to penetrate. Margarine contains unsaturated fats. When it gets colder it remains easy to pass a knife through.
Ms Onyemaobi says cold-tolerant lines can be thought of like margarine. They have higher levels of unsaturated lipids (fats) that allow cell membranes to maintain fluidity.
“If we can prove this and build a reliable screening method, we will not need to rely on field work that can be hard to control,” she says.
Dr Berger says the team’s other cold-tolerance work involves screening the crosses made by Curtin University. “They made crosses of wild and domestic species to expand the crops’ genetic diversity. We’ve found that some of this material has a reasonable tolerance to the cold too. So, we are putting them out in the field to screen them and create something that breeders can use.”
He says the work is a real mix of discovery and production. “Australia has a good history of taking a scientific approach like this.”
Acidic soils
In Australia, where surface and subsoil acidity compromises about half of all agricultural land, tolerant varieties are vital because these conditions limit chickpea production.
Murdoch University’s Dr Wendy Vance, who is leading the research, says that on most acidic soils, toxic levels of aluminium and manganese – along with phosphorus deficiency – are found.
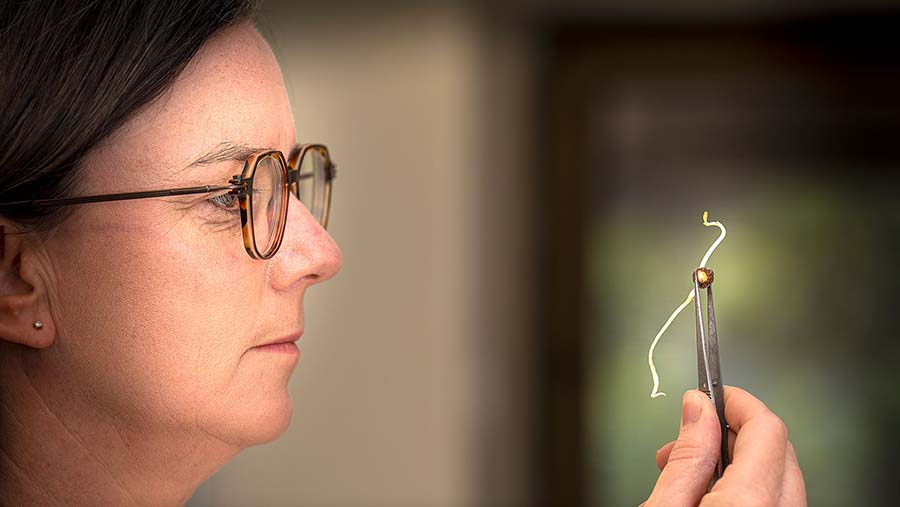
Murdoch University’s Dr Wendy Vance is leading chickpea tolerance research. Photo: Evan Collis
“Chickpeas do not grow productively on acidified loams and clays where pH is below 6. However, the limiting factors to chickpea growth when soil pH declines are not well defined.”
Research already undertaken suggests that aluminium toxicity is not likely to be the major limiting factor. “While aluminum toxicity is most common in severely acid soils, manganese is a likely growth-limiting factor in moderately to strongly acid soil. And low pH itself is also a limitation for chickpeas.”
With this in mind, her colleague Dr Karthika Pradeep has developed a protocol for screening chickpeas to understand tolerance levels to manganese.
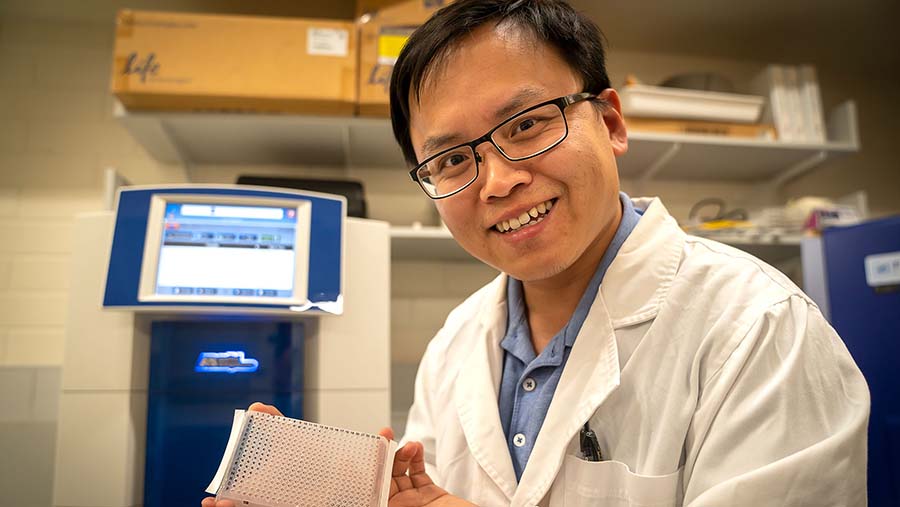
Yong Jai, post-doctoral fellow at Murdoch Uni, has been working with Dr Karthika Pradeep on the chickpea trait’s gene expression. Photo: Evan Collis
“Screening for tolerance to acidic soils previously focused on aluminium toxicity. However, screening only for aluminium tolerance would be incomplete without ensuring that the germplasm also expressed tolerance to manganese toxicity,” Dr Vance says.
“We wanted to determine the concentration of manganese that shows better discrimination among chickpeas accessions and, also, to test whether the methodology works for wild relatives as well.”
Results for a 14-day solution screen differentiated between tolerant and intolerant genotypes, showing the methodology worked and could be used to improve testing for manganese resistance.
Dr Vance says the work has the scope to broaden growing areas. “In addition to searching for tolerance to strongly acid soils, there is an opportunity to broaden the present range of soils suitable for chickpeas by improving tolerance to moderately acid soils.”
More information: Kristy Hobson, kristy.hobson@dpi.nsw.gov.au; Nathan Craig, eo@wmgroup.org.au; Richard Devlin, richard@livingfarm.com.au; Mark Seymour, mark.seymour@dpird.wa.gov.au; Robert Lee, robert.lee@curtin.edu.au; Lars Kamphuis, lars.kamphuis@curtin.edu.au; Jens Berger, jens.berger@csiro.au; Wendy Vance, w.vance@murdoch.edu.au

























































